What Happens to the Hexagonal Open Structure of Ice When Sufficient Pressure Is Applied to It?
Natural snow and ice on Earth occur as hexagonal ice (ice Ih), as evidenced in the six-fold symmetry in ice crystals grown from h2o vapor (that is, snowflakes).
'Why do snowflakes always autumn as flat structures with six corners?'
'Every crystal was a masterpiece of design and no 1 design was always repeated'
Snowflakes described by W. A. Bentley. 1925
Glacier iceberg

Hexagonal ice
In that location are four different naturally occurring morphological forms of hexagonal water ice; snowfall, firn (multi-year snow), freshwater ice, and sea water ice [3584]. Hexagonal ice ([1969], water ice Ih i see Stage Diagram), is in Infinite group P6three/mmc, yard 194; symmetry D6h, Laue class symmetry 6/mmm; coordinating to β-tridymite silica or lonsdaleite. Information technology possesses a relatively open low-density structure, where the packing efficiency is depression (≈ 1/3) compared with elementary cubic (≈ 1/2) or face-centered cubic (≈ three/4) structures a (and in contrast to face-centered cubic close-packed solid hydrogen sulfide).
Hexagonal ice unit cell
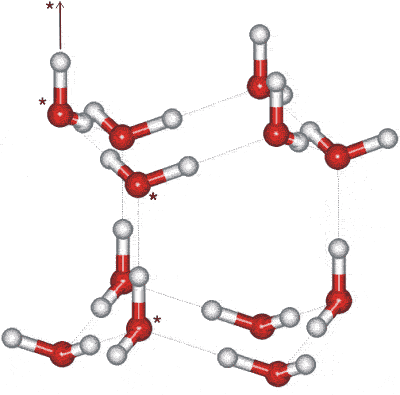
The crystals may be thought of as mirror images of corrugated oxygen sheets lying on elevation of each other. The basic structure consists of a hexameric box where planes consist of chair-grade hexamers (the two horizontal planes, opposite) or boat-course hexamers (the three vertical planes, opposite). In contrast, cubic ice contains solely chair-form hexamers. In this diagram, the hydrogen bonding is shown ordered whereas, in reality, it is random, b as protons can motility between (ice) water molecules at temperatures higher up near 5 Grand [1504]. Indeed, it is probable that the protons in ice behave like a breakthrough liquid in a constant tunneling flux [2301]. This thought is reinforced by neutron handful experiments showing a proton scattering density midway between oxygen atoms [1969], indicating proton delocalization and concerted proton movement.
The water molecules have a staggered hydrogen bonding concerning 3 of their neighbors in the plane of the chair-course hexamers. The fourth neighbor (shown as vertical links contrary) has an eclipsed arrangement of hydrogen bonding.
Hexagonal ice lattice
There is a slight divergence from ideal hexagonal symmetry. The unit cell c is 0.3 % shorter in the c-direction (in the direction of the eclipsed hydrogen-bonding, shown as vertical links in the figures). This gives ascent to reduced compressibility in the c-direction [3152]. The unit cell may be considered every bit a group of four molecules (iii shown starred in the higher up-right figure, with the fourth linked as indicated). The crystallographic c-axis is in the vertical direction (encounter correct). The hexagonal crystal has unit of measurement jail cell dimensions 4.5181 Å (a) (the lateral lattice spacing) and 7.3560 Å (c) (90º, 90º, 120º, four molecules, at 250 K) [382] (the interlayer spacing is one-half this = three.6780 Å); with average nearest O···O altitude = 2.76 Å, with H···O and O-H bail distances of one.75 Å and 1.01 Å respectively. In a perfect crystal, the 'c' cell parameter would be 2√(2/three) times the 'a' cell parameter (7.3780 Å). The cell dimensions for D2O are 4.5216 Å (a) and 7.3627 Å (c) [382], with its c-axis very slightly less affected. There appears to be a slight variation in the c/a axial ratios of D2O ice Ih beneath 160 K consequent on the partial ordering of the hydrogen bonds (see ice Eleven) that depends upon both the preparation method and the thermal history of the sample [3596].
All molecules experience identical molecular environments. There is enough space within each box to hold an interstitial water molecule. Although this is more often than not not thought to occur, interstitial water molecules have been institute past neutron diffraction of effectively powdered ice [154]. Modeling in 2015, found that the O···O–H angle in ice, which would exist 0° for a linear hydrogen bond, is more disordered in ice than in the liquid [4016].
Hexagonal ice has triple points with liquid and gaseous water (0.01 °C, 612 Pa), liquid water and water ice-3 (-21.985 °C, 209.9 MPa), water ice-xi and ice-2 (-199.eight °C, 70 MPa), and ice-2 and water ice-three (-34.vii °C, 212.nine MPa). The dielectric abiding of hexagonal ice is 97.five [94].
The melting curve for hexagonal ice is given by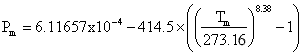 MPa [1320] g. The IAPWS release on the equation of state for hexagonal water ice is bachelor. Some simple equations relating the variation of the physical properties with the temperature of hexagonal water ice and its aqueous slurries are bachelor [1665]. h The experimental rubberband constants of water ice Ih have been compared with semi-empirical water models [3585] and density functional theory [3897]. The hardness of water ice varies with the temperature, increasing from about or below that of gypsum (≤2 on Mohs scale) at 0 °C to about that of feldspar (half-dozen on Mohs scale) at -80 °C [2097], an anomalously large alter in absolute hardness (>24 times) with temperature.
MPa [1320] g. The IAPWS release on the equation of state for hexagonal water ice is bachelor. Some simple equations relating the variation of the physical properties with the temperature of hexagonal water ice and its aqueous slurries are bachelor [1665]. h The experimental rubberband constants of water ice Ih have been compared with semi-empirical water models [3585] and density functional theory [3897]. The hardness of water ice varies with the temperature, increasing from about or below that of gypsum (≤2 on Mohs scale) at 0 °C to about that of feldspar (half-dozen on Mohs scale) at -80 °C [2097], an anomalously large alter in absolute hardness (>24 times) with temperature.
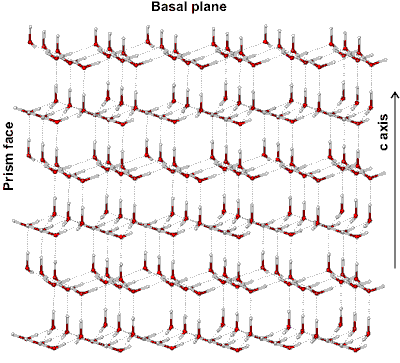
Water ice crystal structure showing iii crystal planes
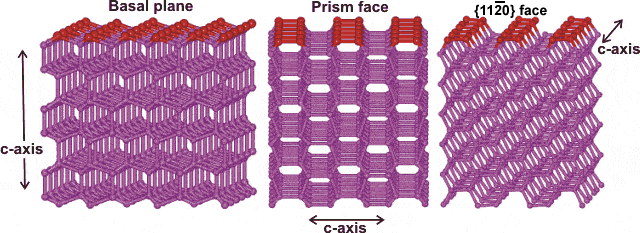
The prism face water molecules extend slightly farther (0.39 nm) than the basal plane water molecules (0.37 nm) [3385]
Hexagonal ice crystal showing
the chief crystal faces
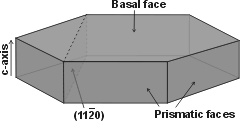
ice crystals giving miller indices
(x,y,z) of faces; from [2304]
![ice crystals giving miller indices (x,y,z) of faces; from [2304] ice crystals giving miller indices (x,y,z) of faces; from [2304]](https://water.lsbu.ac.uk/water/images/ice-miller.gif)
Hexagonal ice crystals grade hexagonal plates and columns where the elevation and bottom faces are basal planes {0001} with enthalpy 5.57 �J ˣ cm−2 [3019], and the half dozen equivalent side faces are called the (primary) prism faces {10i0}di with enthalpy five.94 �J ˣ cm−2 [3019]. Secondary prism faces {xiii0} dii with enthalpy 6.90 �J ˣ cm−2 [3019] may be formed downward the planes formed past the sides of the chair structures.
Hexagonal ice shows an dissonant reduction in thermal conductivity with increasing force per unit area (equally practise cubic ice and depression-density baggy ice) only is different from most crystals. This is due to changes in the hydrogen bonding decreasing the transverse sound velocity [617]. The experimental 2D IR spectra of D2O isotopically pure water ice Ih, ice II, water ice V, and water ice Xiii have been compared [3121].
The hydrogen bonding in the surface of hexagonal ice is predicted to be more ordered than that within the bulk structure [1529]. The 10-ray assimilation spectra determined for preparations of hexagonal ice, although qualitatively like, give differences in the values of pre-edge, master-edge, and postal service-edge intensities and their relative ratios. This may be due to different preparation procedures giving possible contamination by cubic ice or low-density amorphous (LDA) water ice [3558]. Methods have been published describing how to ready big water ice crystal samples and whatever desired confront of ice [3019].
Phase-resolved sum-frequency-generation vibrational spectroscopy has shown a structural asymmetry between the summit ii layers (L1 top layer of water hexagons, L2 second layer of h2o hexagons) in the subsurface hydrogen-bonding of the basal face of hexagonal ice. Accepted hydrogen bonds in the top surface hexagons (L1 O···H-O L2) are stronger than the accepted hydrogen bonds in the second layer hexagons to the top layer (L1 O-H···O L2) [3100].
The electric properties of water ice (e.1000., electrical conductivity) have been explained using the two types of free-moving defects in water ice; the presence of ions (HiiiO+ and OH−) and Bjerrum defects (wrongly oriented water molecules) [3691]. The structural mechanisms of water ice deformation have been studied [3158].
Interactive structures of hexagonal water ice (Jmol) are available. [Back to Peak ![]() ]
]
Water ice nucleation and growth
How ice crystals form has relevance to cloud physics, cryopreservation, and the prevention of icing on structures, such as aircraft wings, bridge cables, and wind turbines. The progress in ice nucleation [3492, 4333] has been reviewed. In the atmosphere, water ice-nucleating particles are often naturally mixed with a soluble material. Small concentrations of solutes (0.015 Thou), with predicted minimal colligative effect (freezing point depression < 0.1 °C) and negligible bear on on h2o action, tin influence the heterogeneous nucleated freezing using suspended particles of feldspars or quartz. Surprisingly the freezing points may be increased by up to 3 °C (using ammonium salts) or decreased (using alkali metal halides) by up to viii °C for identical nucleating rock particles [3379], with these temperature changes relative to the nucleation without whatever solute present. Crystallization from supercooled liquid h2o involves ordering the molecules and adopting their ice lattice positions. The ice nuclei originate within immobile regions of the supercooled water [3523].
The minimum number of water molecules necessary for ice nucleation in pure water was offset stated to be the same (i.e., 275±25) every bit that required for a complete icosahedral cluster (i.eastward., 280) [1931]. More recent piece of work has suggested that just well-nigh ninety molecules are necessary [3801], with water clusters with less than ~ 150 molecules oscillating betwixt the liquid and crystalline (a mixture of structurally like ice Ih and Ic species) states. Ice nucleation occurs profoundly enhanced, by 1010, at the air-h2o surface rather than within bulk water [914]. Ice crystal growth depends on the different growth rates on each facet, with facet nucleation rates dependent on their different step energies. The secondary-prism face is near stable, followed by the primary-prism face and then the hexagonal basal face [2193]. This gives ascent to the hexagonal 'snowflake' shapes (come across the height of the page, right), with their great variety due to crystal defects. The physical dynamics of ice crystal growth have been comprehensively described [3019]. Water ice nucleation may exist triggered by negative pressures that occur locally and briefly when h2o is stretched due to mechanical daze or ultrasonic waves [3123]. Ice nucleation in atmospheric clouds may be through the stacking-disordered ice.
H2o must exist prevented from freezing when cryopreserving biological samples, food, and organs [2853]. This is by and large accomplished by fast cooling rates, the utilize of small-scale samples and cryopreservation agents, and increasing pressure level to vanquish ice nucleation and avoid cell damage [2821]. The ice/liquid interfacial energy increases from about thirty mJ ˣ m−2 at ambient pressure to 40 mJ ˣ yard−2 at 200 MPa, indicating the reason for the reduction of the water ice nucleation with increasing pressure [2821].
The growth of ice on freezing
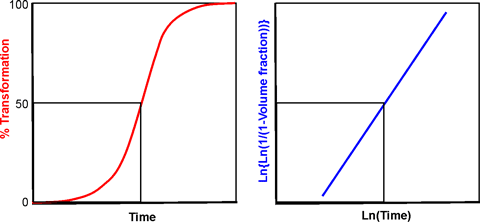
The crystallization of water may exist described by the Johnson-Mehl-Avrami-Kolmogorov (JMAK) phase change equation. j The transformation starts at a slow rate where nuclei form, followed by the rapid growth of the crystals.
Needle ice in Yellowstone Park

Hexagonal water ice crystals may class past (slowly) growing in the direction of the c-centrality (Due south1 water ice). Examples are the within of vertical freezing pipes, where water ice crystals abound downwardly vertically from crystal platelets nucleated on yet water with their c-axes vertical, and where sideways growth is prevented merely axial growth immune. Alternatively, they may grow more chop-chop from the prism faces (S2 ice), as at the disturbed randomly-directed surface of chop-chop freezing or agitated lakes. Growth from the {1 1 -2 0} faces is at to the lowest degree as fast as that from the prism faces, simply such growth turns these faces into prism faces [827]. Water ice crystal growth information has been critically examined [3046]. The relative speeds of this crystal growth of the different faces depend on the power of these faces to grade greater extents of cooperative hydration. The temperature of the (supercooled) surrounding water determines the degree of branching in the ice crystal. Crystal growth is limited by the rate of diffusion at a low degree of supercooling (i.e., < two °C; giving rise to more branching) but express by the kinetics of growth at higher degrees of supercooling (i.e., > iv °C; giving rise to needle-similar growth) [883]. The hexagonal crystal structure, the dissimilar growth characteristics of the crystal faces, and the temperature of the surrounding (supercooled) h2o are behind the flat six-pointed shapes of snowflakes [1916].
Vapor pressure level of ice
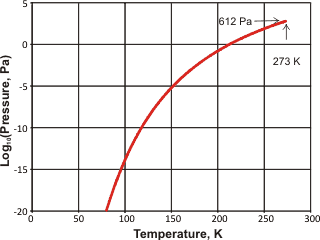
The vapor force per unit area of ice is shown right [IAPWS]. Water ice nucleation in the atmosphere profoundly affects deject germination and backdrop. Feldspars, found in desert dust that enters the atmosphere by the million tons a year, are important nucleation agents. Computer simulations accept indicated that this is due to the nucleation of the prismatic crystal planes of ice on the loftier-energy (100) surface planes of feldspar [2863].
Solutes (except for the very pocket-sized helium [2204] and hydrogen [2205] molecules that can fit into the interstitial sites) cannot be incorporated into the crystalline water ice Ih construction at ambience pressure f but are expelled to the surface or to the baggy ice layer between the microcrystalline ice crystals [3838]. The microscopic mechanism for ion rejection during freezing has been investigated.
The hydration energy for the ion-water interaction was found to be stronger than that betwixt ions and ice, so causing ion rejection [4300]. Ion rejection may be used when purifying water (for example, degassing or desalination [2851] ) using successive freeze-thaw cycles. Some solutes (for example, chaotropic ions such as NHiv + and Cl−) are incorporated more than easily into freezing ice than others (for example, kosmotropic ions such as Na+ and And soiv 2−), removing them from the remaining thin films of liquid between the crystals. This may result in the electrical charging of the surface due to surface h2o dissociation balancing the remaining charges (which also may give rise to electromagnetic emission) and changes in the pH of the residual liquid films (for instance, (NH4)twoTheniv becomes more acidic [1010]. NaCl solutions becomes more than alkaline metal [1089, 4155], reflecting preferential incorporation of the different ions into the ice. Between the pure ice particles, the concentration of salts in aqueous channels gives rise to efficient ionic transport, with the ice becoming a solid-state ionic conductor [3755].
Views perpendicular to the faces of the ice (1h)
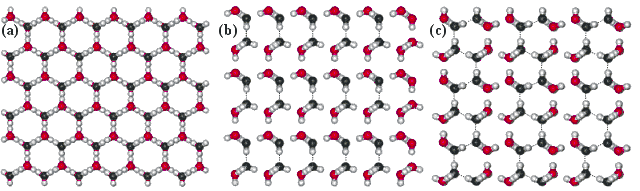
Views perpendicular to the water ice (1h) crystal's faces prove the next layer fastened (with O-atoms black). (a) Slow growing basal {0 0 0 1} face up (viewing downwards the c-axis); where simply isolated water molecules attach. (b) faster growing {1 0 -1 0} prism face up (viewing down the a-centrality), where pairs of newly-fastened water molecules may grade hydrogen bonds to each other; 1 hydrogen bail/ii water molecules. (c) Fastest growing {i i -2 0} (secondary prism) face, where chains of newly-attached water molecules may cooperatively hydrogen bond to each other; ane hydrogen bail/water molecule. These form ridges which divide and encourage conversion into two prism faces. The radial distribution functions are shown elsewhere.
The rationale behind the extensive range of snow crystal forms has been explained every bit due to changes in temperature and humidity during their formation. The crystal growth rates of the basal and the prism faces exhibit a complex temperature dependence within clouds and the atmosphere, and their prevalences cantankerous over upwards to three times in a range betwixt 0 °C and -40 °C [4011].
The development of ice-phobic surfaces is very important for transportation systems (e.g., plane wings), power systems (e.g., power cables), and free energy systems (eastward.thousand., electricity manual). Water ice-phobic surfaces should accept a depression freezing temperature, low water ice accession charge per unit, depression ice adhesion, and long-term immovability [3611].
The freezing of h2o in soap bubbling is different from that in liquid h2o with many ce crystals growing iat the same time and swirling effectually the bubbles [3677].
[Dorsum to Peak ![]() ]
]
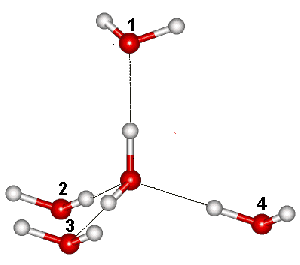
I (1&3) of the six arrangements of hydrogen bonds
Nothing-point entropy
The zero-indicate entropy may be defined as
S 0 = thouB ˣ Ln(Northward E0)
where kB is the Boltzmann abiding, N East is the number of configurations at free energy Eastward, and E0 is the lowest free energy. This value for the entropy of hexagonal water ice at zero kelvin does not break the third law of thermodynamics "The entropy of a perfect crystal at accented zilch is exactly equal to zero" as the hexagonal ice crystals are non perfect, having disordered hydrogen-bonding.
In hexagonal ice, the hydrogen bonding is random and rapidly changing (while obeying the 'ice rules'). These structures are not precisely equal in free energy but spread out over many energetically shut states. The zero-point entropy is entropy (disorder) that would remain even if the material could be cooled to absolute zero ( 0 Chiliad = −273.15 °C). This disorder gives rise to an experimental nothing-point entropy for hexagonal ice of 3.41 (± 0.2) J ˣ mol−1 ˣ Yard−i. An extra disorder at interfaces and defects gives rise to a wide range of bounden energies [4378].
Theoretically, it should be possible to summate the cypher-point entropy of the known ice crystals with far more than accuracy (neglecting flaws, defects, and the spread of free energy levels) than determining information technology experimentally. However, their verbal calculation has been found to be in demand of cleverer mathematicians than having, and then far, tackled the problem.
Linus Pauling estimated the number of hydrogen-bonded configurations of water ice = R ˣ Ln(iii/ii) = 3.371 J ˣ mol−i ˣ K−one (0.99 ˣ the experimental value and the lower bound of the exact value). Pauling used the number of configurations equaling (6 ˣ ¼)N, where North is the number of molecules. The (half dozen ˣ ) term is due to six possible arrangements of the hydrogen atoms along with the 4 hydrogen bonds around each oxygen atom (ane&two, 1&3, one&4, 2&3, 2&4, 3&4, see right). The ( ˣ ¼) term is due to two of the four bonds connecting the oxygen to its four neighbors are already occupied by their neighbors' protons, and the probability is 1-half that a particular bond does not already possess a hydrogen atom. The probability that ii selected bonds are both complimentary of protons is (½ ˣ ½) = (¼).
Pauling'south estimate makes no assart for the many correlations introduced from the airtight hydrogen-bonded loops. The rationale is that the chances of a bond being allowed in a ring of hydrogen bonds are slightly greater than ½ every bit the linkages are correlated. The hydrogen bond being donated from the last water molecule in the ring of h2o molecules to the first molecule in that band finds a water molecule with 2/3 free space rather than 2/4. Thus the (½ ˣ ½) term is an underestimate, and Pauling'south approximate is low; see [717] for farther discussion of this point. A better judge of the zilch-point entropy, from averaging multiple simulations, is R ˣ Ln(1.507117) = 3.413 J ˣ mol−1 ˣ K−1 [1090]; about 1% higher. Nevertheless, the verbal balance entropy of Ice Ih remains unknown, with a 2020 calculated value of three.41449 J ˣ mol−1 ˣ K−1 [4133].
Although the proton order in bulk ice is matted, the surface of the ice probably prefers proton order in terms of stripes of dangling H-atoms and O-lone pairs [1551]. The nix-indicate entropy of ices with ordered hydrogen bonds (such every bit water ice-two) is aught. The zero-point entropies (ZPE, J ˣ mol−ane ˣ Grand−1) of other ices have been found [3107] to be
| Social club Ih = Ic = Ice Vii < Ice III < Clathrate I < Clathrate Two < Water ice V < Clathrate H < Water ice Vi | |||||||||
| ZPE | 3.41251 | three.41251 | 3.41251 | 3.41975 | iii.43191 | 3.43285 | 3.43433 | 3.43834 | three.50082 |
| Ratio % | 100.00 | 100.00 | 100.00 | 100.21 | 100.57 | 100.lx | 100.64 | 100.76 | 102.59 |
[Dorsum to Top ![]() ]
]
Footnotes
a The exact packing efficiency for ice Ih is depression
≈ 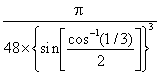 ≈ 0.34,≈ 1/iii
≈ 0.34,≈ 1/iii
compared with the simple cubic
![]() = 0.5236,≈ 1/2
= 0.5236,≈ 1/2
or the torso-centered cubic
![]() = 0.6802,≈ ii/3
= 0.6802,≈ ii/3
or the face-centered cubic and hexagonal shut-packed
![]() = 0.7408,≈ 3/4
= 0.7408,≈ 3/4
structures [811]. [Back]
b In all these structural diagrams, the hydrogen bonding is ordered whereas, in reality, it is random and rapidly changing (obeying the 'ice rules'). Equally the H-O-H angles are about 106.6º [717], the hydrogen bonds are non straight (although shown so in the figures). Although each water molecule is shown symmetrically situated, thermal movement causes a broad range of the hydrogen bond energies giving noticeable instantaneous asymmetry effectually the crystallographic sites [2095]. [Back]
d (i) In that location are six prism face planes around the hexagonal crystal; going anticlockwise {0 1 -i 0} {-1 1 0 0} {-ane 0 1 0} {0 -1 1 0} {i -1 0 0} {1 0 -1 0}. (ii) There are six such secondary prism face up planes across similar diagonals in the hexagonal crystal; going anticlockwise {1 1 -2 0} {-ane ii -ane 0} {-2 one one 0} {-1 -1 2 0} {1 -2 ane 0} {ii -one -ane 0}. [Dorsum]
f All the same, the shut match of the crystal lattice constants betwixt hexagonal ice and hexagonal β-AgI makes the AgI an effective ice-nucleating amanuensis [2833]. Similarly, the hydrogen-bonded salt NH4F (with both NH4 + and F− existence of similar size to H2O molecules and giving tetrahedrally directed hydrogen bonds, with hexagonal unit jail cell dimensions 4.37 Å (a) and 7.17 Å (c)) can likewise form thoroughly mixed hexagonal crystals [1568]. The poor solubility of other solutes in hexagonal water ice does not extend to the high-pressure level ices, where salts may be incorporated into the crystal lattice. [Back]
thou There seems to be an mistake in the published equation, where the denominator is given as 273.15. [Back]
h The variation of some physical backdrop of ice may be given in terms of the temperature (T, °C) [1665]:
Density of ice (kg ˣ m−3) = 917 – 0.13 ˣ T
Specific heat of ice (kJ ˣ kg−1 ˣ K−i) = 2.12 + 0.008 ˣ T
Thermal conductivity of ice (W ˣ m−1 ˣ K−1) = ii.21 – 0.012 ˣ T
Enthalpy of ice (kJ ˣ kg−1) = –332.4 + T ˣ (2.12+0.008 ˣ T)
Melting pressure (MPa) = –395.2 ˣ {(T/273.15)nine – 1} T in K
Note that ice oftentimes has included gas pockets inside its structure and hence has an apparently lower density and altered physical properties.
[Dorsum]
i The I in 'ice Ih' is the Roman numeral for one, as this was the first water ice establish. [Back]
j M. Avrami, Kinetics of stage change. I. General theory,Journal of Chemic Physics,7 (1939) 1103-1112; M. Avrami, Kinetics of phase change. 2. Transformation-time relations for random distribution of nuclei,Journal of Chemical Physics,8 (1940) 212-224; M. Avrami, Kinetics of stage alter. Iii. Granulation, phase change, and microstructure,Journal of Chemic Physics,nine (1941) 177-184. [Back]
kPsixthree/mmc means having a six-fold screw axis (rotation around an axis in addition to a translation along the axis); the structure repeats itself iii-times during a consummate 360° rotation; the 'thousand's stand for mirror planes perpendicular to the basal aeroplane and parallel to the 'c' axis, and with the c in 'mmc' standing for the glide plane. [Back]
fredericksvizienteling.blogspot.com
Source: https://water.lsbu.ac.uk/water/hexagonal_ice.html
0 Response to "What Happens to the Hexagonal Open Structure of Ice When Sufficient Pressure Is Applied to It?"
Post a Comment